Molecular mechanism of action of K(D)PT as an IL-1RI antagonist for the treatment of rhinitis
Abstract
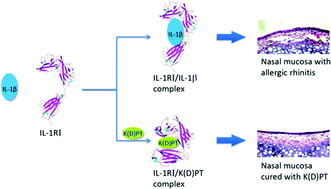
Background: Interleukin-1 receptor type I (IL-1RI) is critical for both innate immunity and inflammation. IL-1RI stimulates thymocyte proliferation and the release of several interleukin cytokines. These properties have increased interest in targeting IL-1RI for the treatment of inflammatory diseases. Here, an IL-1RI antagonist, K(D)PT (Lys-D-Pro-Thr), was tested in an allergic rhinitis model. The mechanism of action was then investigated. Methods: HEK293/IL-1RI cells and an allergic rhinitis animal model were treated with K(D)PT to evaluate its therapeutic effects. Fifty nanosecond (ns) molecular dynamic (MD) simulations were performed on the K(D)PT/IL-1RI complex, unliganded IL-1RI, and the IL-1β/IL-1RI complex to explore the mechanism of action of K(D)PT. Results: K(D)PT down-regulated the IL-1RI-mediated induction of IL-2 and IL-4 mRNA expression by IL-1β in HEK293/IL-1RI cells. In addition, nose itching was alleviated in mice treated with K(D)PT. Serum levels of IL-2 and IL-4 as well as eosinophil infiltration were also reduced. The data suggested that IL-1RI was highly expressed in the nasal mucosa of mice with allergic rhinitis. MD simulations revealed the following: (1) IL-1RI remains in the open conformation in the IL-1RI/IL-1β complex; (2) in unliganded IL-1RI, domains I and III randomly moved closer and apart without any significant energetic changes; (3) K(D)PT locks the C- and N- terminals of IL-1RI by forming hydrogen bonds with both terminals to adopt a closed conformation and consequently minimizes the system energy. Conclusions: IL-1RI antagonist K(D)PT effectively treated allergic rhinitis. The molecular mechanism of action indicated that K(D)PT connects the C- and N- terminals of IL-1RI via hydrogen bond formation to establish a stable conformation and consequently minimize the system energy of IL-1RI.

 Please wait while we load your content...
Please wait while we load your content...