Controlled RAFT synthesis of side-chain oleic acid containing polymers and their post-polymerization functionalization†
Abstract
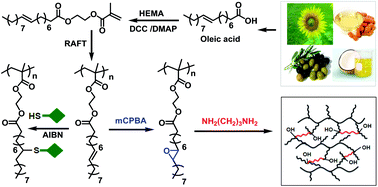
This study provides new insights into the design and controlled synthesis of side-chain unsaturated fatty acid containing polymers, with specific attention to facile post-polymerization modification reactions. The methacrylate derivative of oleic acid, 2-(methacryloyloxy)ethyl oleate (MAEO), has been synthesized and polymerized by the reversible addition-fragmentation chain transfer (RAFT) process to obtain poly(2-(methacryloyloxy)ethyl oleate) (PMAEO) with controlled molecular weight, narrow molecular weight distribution (Đ) and known chain ends. The PMAEO is capable of subsequent chain extension to form well-defined block copolymers via the RAFT technique. Double bonds in the oleate side-chains in PMAEO are further reacted with various organic thiol compounds via thiol-ene based thia-Michael addition reactions in the presence of 2,2′-azobisisobutyronitrile (AIBN) as radical source at 60 °C in tetrahydrofuran (THF) solvent. 1H NMR spectroscopy indicated quantitative conversion of double bonds with ethanethiol, butanethiol, dodecanethiol and 3-mercaptopropanoic acid, whereas 2-mercaptoethanol gave ∼90% thioether product. The side-chain double bonds of PMAEO were quantitatively epoxidized using meta-chloroperbenzoic acid (mCPBA) at room temperature in dichloromethane, which was further cross-linked by 1,3-diaminopropane. Therefore, side-chain oleate pendants are ideal for various post-polymerization modifications to prepare renewable resource derived controlled macromolecular architectures with potential practical applications in fields such as paints, adhesives, electrical insulators, thermoplastics, etc.

 Please wait while we load your content...
Please wait while we load your content...