A novel quinone/reduced graphene oxide composite as a solid-phase redox mediator for chemical and biological Acid Yellow 36 reduction†
Abstract
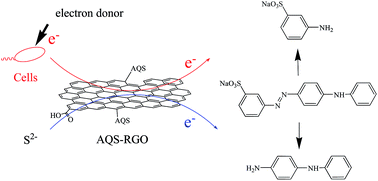
A novel quinone-modified graphene material was developed to enhance the catalytic performance of graphene for the transformation of environmental pollutants. Graphene oxide (GO) was first reduced with diethylenetriamine and the obtained NH2-RGO was further covalently modified with anthraquinone-2-sulfonic acid (AQS). The prepared AQS-modified RGO (AQS-RGO) was characterized by XPS, IR and SEM, respectively. The catalytic performance of AQS-RGO was investigated during azo dye Acid Yellow 36 (AY 36) decolorization. It was shown that pH and temperature had significant effects on AY 36 chemical decolorization by Na2S. At the pH of 6.0, AQS-RGO exhibited better catalytic performance compared with NH2-RGO due to the lower activation energy of the AQS-RGO amended system. AY 36 bio-decolorization could be enhanced in a dose-dependent manner with AQS-RGO. In the presence of 25 mg L−1 AQS-RGO, the AY 36 bio-decolorization rate was 6.7-fold higher than that mediated by NH2-RGO, indicating that AQS-RGO is a better electron-transfer mediator. Based on the above results, the electron transfer pathways of AQS-RGO-mediated AY 36 decolorization were proposed. These findings indicate that the application of AQS-RGO is a feasible method to enhance the treatment of azo dye-containing wastewater.

 Please wait while we load your content...
Please wait while we load your content...