Antioxidant activity of fraxetin and its regeneration in aqueous media. A density functional theory study†
Abstract
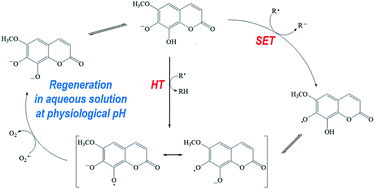
In this work, we have carried out a quantum chemistry and computational kinetics study on the reactivity of fraxetin towards two peroxyl free radicals (˙OOH and ˙OOCH3), in aqueous and lipid simulated biological environments. We have considered three reaction mechanisms: hydrogen transfer (HT), radical adduct formation (RAF), and single electron transfer (SET). Rate constants and relative branching ratios for the different paths contributing to the overall reaction, at 298.15 K, are reported. In aqueous media, fraxetin exists in two forms, depending on pH. Neutral fraxetin reacts mainly through the HT mechanism, while the deprotonated fraxetin reacts mainly through the SET mechanism. The overall reaction rate constants are 3.99 × 108 and 2.76 × 109 M−1 s−1 for reaction with ˙OOH and ˙OOCH3 peroxyl radicals, respectively. In addition, we have shown that fraxetin is a versatile antioxidant in aqueous media, since it has a great scavenger activity towards other free radicals, under the same conditions. Furthermore, the possible regeneration of fraxetin after scavenging a first radical was investigated in aqueous solution at physiological pH. It was found that regeneration is very likely to occur, which suggests that this compound has the ability to scavenge several radical equivalents (two per cycle), under such conditions. In lipid media, fraxetin reacts with the peroxyl radicals only through the HT mechanism, and the calculated reaction rate constants are 2.43 × 104 and 2.81 × 103 M−1 s−1 for ˙OOH and ˙OOCH3 radicals, respectively.

 Please wait while we load your content...
Please wait while we load your content...