Microwave-assisted palladium-catalyzed highly regio- and stereoselective head to head dimerization of terminal aryl alkynes in water†
Abstract
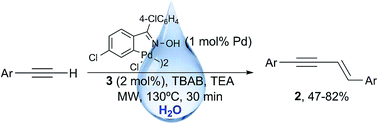
A highly regio- and stereoselective oxime palladacycle/imidazolinium-catalyzed head to head dimerization of terminal aryl alkynes in water is presented. The reaction, which is carried out at 130 °C under microwave irradiation in the presence of 1,3-bis-(2,6-diisopropylphenyl)imidazolinium chloride as ligand, triethylamine as base, and TBAB as surfactant, allows the synthesis of (E)-1,4-enynes as single stereoisomers in good isolated yields.

 Please wait while we load your content...
Please wait while we load your content...