Facile synthesis of boronic acids on a BODIPY core with promising sensitivity towards polyols†
Abstract
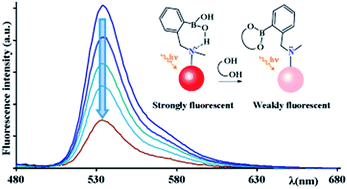
An efficient strategy for the synthesis of o-((ethyl)aminomethyl) phenyl boronic acid anchored on a BODIPY core is reported. Kinetic studies highlight the association with diols and fructose; BODIPY fluorescence is quenched upon substrate binding, showing an unusual turn-off response.

 Please wait while we load your content...
Please wait while we load your content...