Solid Lewis acidity of boehmite γ-AlO(OH) and its catalytic activity for transformation of sugars in water
Abstract
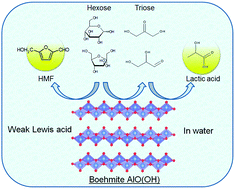
Aqueous-phase transformation of C3- and C6-sugars toward valuable intermediates such as lactic acid and 5-hydroxymethylfurfural (HMF) using boehmite, an abundant, inexpensive and simple aluminium oxide hydroxide γ-AlO(OH) was demonstrated. Pyridine-adsorbed Fourier transform infrared (FTIR) spectroscopy and trimethylphosphine oxide-adsorbed 31P magic angle spinning (MAS) nuclear magic resonance (NMR) revealed that boehmite had no Brønsted acid sites and a small amount of weak Lewis acid sites. The amount of Lewis acid sites and the initial reaction rate for lactic acid synthesis from dihydroxyacetone increased with increase of calcination temperature of boehmite whereas the selectivity for lactic acid remained unchanged and turnover frequency decreased. Boehmite afforded lactic acid selectively from aqueous C3-sugars solution with the yields of 32% and 42% from dihydroxyacetone and glyceraldehyde, respectively, which were higher than those of hydrotalcite, titanium oxide, tin oxide and tungsten oxide. Moreover, boehmite gave both lactic acid and HMF simultaneously from aqueous glucose solution as well as fructose. The total yields of lactic acid and HMF from aqueous glucose solution reached 40%. Boehmite was found to catalyse a variety of reactions including isomerization, retro-aldol condensation, dehydration and hydration in water.

 Please wait while we load your content...
Please wait while we load your content...