A novel colorimetric and fluorogenic chemosensor for selective detection of Cu2+ ions in mixed aqueous media†
Abstract
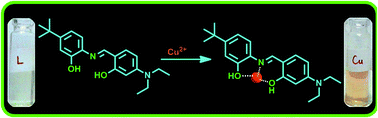
A novel and easy-to-synthesize chemosensor (L) was developed for the selective sensing of Cu2+ ions in mixed aqueous medium. Sensor L showed both colorimetric and spectral (UV-Vis and fluorescence) responses towards Cu2+, which was not affected in the presence of other surveyed metal ions (Na+, Mg2+, Al3+, K+, Ca2+, Fe2+, Co2+, Ni2+, Zn2+, Ba2+, Hg2+ and Pd2+). The 1 : 1 binding stoichiometry between Cu2+ and L was obtained using the Job's plot and ESI-MS analysis. The binding constant (Ka) of 1.56 x 104 M−1 for L–Cu2+ complex was calculated from the Benesei–Hildebrand plot. Further, theoretical calculations were performed using the density functional theory (DFT) method to complement the experimental results.

 Please wait while we load your content...
Please wait while we load your content...