Diels–Alder reactions of pinacol alkenylboronates: an experimental and theoretical study†
Abstract
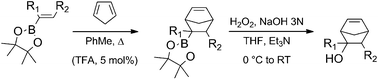
We have studied the Diels–Alder reactions of pinacol alkenylboronates with cyclopentadiene under two different sets of conditions: thermal heating at 170 °C in a pressure tube and with catalytic TFA (5 mol%) at 80 °C. Yields varied significantly from system to system and also for the uncatalyzed and catalyzed methodologies. Moderate to excellent exo-stereoselectivities were obtained in all cases. The theoretical study of the thermal reactions sheds some light on the intriguing substituent effects observed experimentally. A variety of substituted 5-norbornen-2-ols were easily generated by subsequent in situ oxidation of the cycloadducts with alkaline hydrogen peroxide.

 Please wait while we load your content...
Please wait while we load your content...