Design, synthesis and docking studies of some novel isocoumarin analogues as antimicrobial agents
Abstract
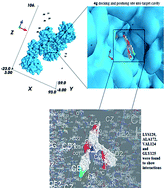
A number of novel isocoumarin analogues have been synthesized by the condensation of homophthalic acid anhydride with different non-steroidal anti-inflammatory drugs (NSAIDs). To investigate the antimicrobial data on a structural basis, in silico docking studies of the synthesized compounds (4a–4g) into the crystal structure of UDP-N-acetylmuramate-L-alanine ligase using an Autodock PyRx virtual screening program were performed in order to predict the affinity and orientation of the synthesized compounds at the activities. UDP-N-acetylmuramate-L-alanine ligase is essential for D-glutamate metabolism and peptidoglycan biosynthesis in bacteria. R2 values showed good agreement with predicted binding affinities obtained by molecular docking studies. The results indicate that the basic nucleic portion of the (4c), (4g), (4f) and (4a) binds into the specificity pocket. In this pocket, the isocoumarin nucleus of these compounds interacts with the amino acid residue of the target. Moreover, it is verified by in vitro antimicrobial screening, in which all of the compounds were active against tested bacterial strains. Among these compounds (4c), (4g), (4f) and (4a) showed good bacterial zone inhibition.

 Please wait while we load your content...
Please wait while we load your content...