Discrete polynuclear manganese nanorods: syntheses, crystal structures and magnetic properties†
Abstract
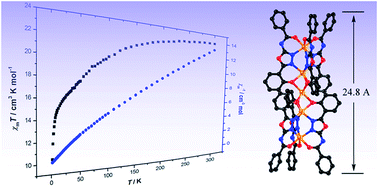
Shape-anisotropic nanomaterials with uniform lengths and widths have attracted much interest but few of them are involved in self-assembled manganese complexes. The combination of two redox-active acylthiosemicarbazide ligands with Mn3+ cations leads to two discrete polynuclear Mn nanorods, pentanuclear [Mn5(L3)6][Mn0.7(DMF)1.4(H2O)2.8] (1a) and trinuclear Mn3(L4)3·2DMF (2), whose L3 and L4 represent N-(5-(2-hydroxyphenyl)-1,3,4-oxadiazol-2-yl)benzamide and N-(5-(2-hydroxyphenyl)-1,3,4-oxadiazol-2-yl)-propionamide, respectively, which are in situ synthesized from their acylthiosemicarbazide derivatives in the presence of Mn3+ cations, while the latter are reduced into divalent ones in rod-like moieties. For each pentanuclear anion in 1a, the charge is balanced by a solvated trivalent manganese cation, which can be replaced by two tetramethylammonium cations to yield pure valence compound 1b. Moreover, the magnetic studies reveal all of them possess antiferromagnetic properties.

 Please wait while we load your content...
Please wait while we load your content...