Kinetics of hydrodeoxygenation of octanol over supported nickel catalysts: a mechanistic study†
Abstract
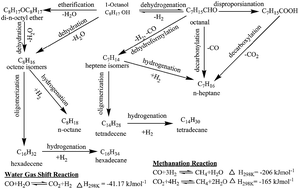
The hydrodeoxygenation (HDO) of 1-octanol as a model aliphatic alcohol of bio-oil was investigated in a continuous down-flow fixed-bed reactor over γ-Al2O3, SiO2, and HZSM-5 supported nickel catalysts in the temperature range of 488–533 K. The supported nickel catalysts were prepared by incipient wetness impregnation method and characterized by BET, XRD, TPR, TPD, H2 pulse chemisorption, and UV-vis spectroscopy. Characterization of supported nickel (or nickel oxide) catalysts revealed existence of dispersed as well as bulk nickel (or nickel oxide) depending on the extent of nickel loading and the nature of the support. The acidity of γ-Al2O3 supported nickel catalysts decreased with increasing the nickel loading on γ-Al2O3. n-Heptane, n-octane, di-n-octyl ether, 1-octanal, isomers of heptene and octene, tetradecane, and hexadecane were identified as products of HDO of 1-octanol. The C7 hydrocarbons were observed as primary products for catalysts with active metal sites such as γ-Al2O3 and SiO2 supported nickel catalysts. However, C8 hydrocarbons were primarily formed over acidic catalysts such as pure HZSM-5 and HZSM-5 supported nickel catalyst. The 1-octanol conversion increased with increasing nickel loading on γ-Al2O3, and temperature and decreasing pressure and WHSV. The selectivity to products was strongly influenced by temperature, nickel loading on γ-Al2O3, pressure, and types of carrier gases (nitrogen and hydrogen). The selectivity to C7 hydrocarbons was favoured over catalysts with increased nickel loading on γ-Al2O3 at elevated temperature and lower pressure. A comprehensive reaction mechanism of HDO of 1-octanol was delineated based on product distribution under various process conditions over different catalysts.


 Please wait while we load your content...
Please wait while we load your content...