Emulsion ionic liquid membranes (EILMs) for removal of Pb(ii) from aqueous solutions
Abstract
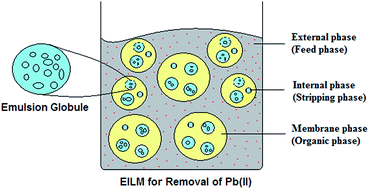
Ionic liquids (ILs) are playing increasingly important roles in the membrane separation processes. The present manuscript discusses the removal of Pb(II) ions from aqueous solution using an emulsion ionic liquid membrane (EILM) process. Initially, the emulsion liquid membrane (ELM) was prepared by stirring strip phase (sulphuric acid) and organic phase (surfactant: span 80, extractant: D2EHPA, diluent: hexane) together under high speed agitation. Note that, the parameters of the ELM process such as emulsification speed, pH of the feed phase, treat ratio, extractant and surfactant concentrations were studied for the maximum removal of Pb(II) ions. The role of IL was explored by adding hydrophobic IL, octylmethylimidazole hexafluorophosphine ([OMIM][PF6]), in the organic phase. The performance of ELM with and without IL was compared on the basis of stability, enrichment factor and the removal efficiency for Pb(II). The results showed that the percentage of Pb(II) extraction was complete by the emulsion membrane with IL (EILM) in comparison to the 97% achieved by neat ELM. Further, the stability and the enrichment factor of the EILM were found to be 2–3 times greater than that of the ELM. The FT-IR spectroscopic analysis revealed that bond interactions between IL and membrane phase components avoided the coalescence of internal phase droplets and enhanced the emulsion stability. The results obtained in this work support the use of the IL [OMIM][PF6] as both a stabilizer and carrier for the overall improvement of the ELM process.

 Please wait while we load your content...
Please wait while we load your content...