Conformational and zwitterionic preferences of N-amidinoglycine: the effect of microsolvation and metal ion addition†
Abstract
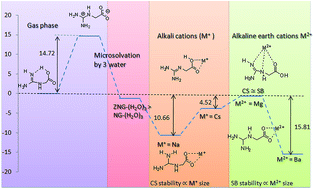
N-Amidinoglycine (NG) is a precursor of creatine, which is the energy source in muscles and plays a vital role in adenosine triphosphate homeostasis. NG possesses the combined structural properties of Gly and Arg. Extensive work on cationic complexation, anionic complexation, microsolvation, excess electron binding, etc. is reported on Gly as well as Arg. Quantum chemical calculations have been performed using B3LYP, BHandHLYP, ωB97XD, M06, M06-2X and MP2 methods, on NG to understand the conformational and zwitterionic preferences under the influence of metal ions (alkali and alkaline earth ions) and water coordination, as well as on the bare structures. NG can have many alternative structures; at least ten structures are possible within 5 kcal mol−1, in the gas phase. NG-1 and NG-2 are highly competitive structures as global minima. The zwitterion (ZNG-1) is about 15 kcal mol−1 less stable than the canonical form (NG-2) in the gas phase although its basicity is high as Arg. This unusual instability of the zwitterionic form of NG may be attributed to the lack of intramolecular self-solvation (multiple hydrogen bonding between the guanidine and carboxylate groups) which is found in Arg. Microsolvation decreases energy differences between canonical and zwitterionic forms of NG, three water molecules are necessary to make them energetically competitive. Computational analysis indicated that the relative stabilities of canonical conformers and zwitterions of NG are very sensitive to their surroundings. Salt bridge structures of NG with alkali and alkaline earth metal cations are stable over charge solvated structures by about 2–8 and 1–14 kcal mol−1, respectively, except in the case of the Be+2 ion.

 Please wait while we load your content...
Please wait while we load your content...