High nuclearity Ni(ii) cages from hydroxamate ligands†
Abstract
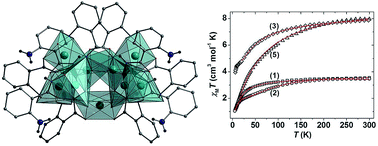
The synthesis, structural and magnetic characterisation of a family of Ni(II) cages built from hydroxamate ligands is presented. Two pentanuclear 12-MCNi(II)-4 metallacrowns [Ni5(L1)4(MeOH)4](ClO4)2·2MeOH (1) and [Ni5(L1)4(py)5](ClO4)2·H2O (2) (where L1H2 = 2-(dimethylamino)phenylhydroxamic acid) share analogous, near-planar {Ni5(L1)4}2+ cores, but differ in the number and nature of the ligands located at the axial Ni(II) sites; the addition of pyridine converting square planar Ni(II) ions to square-based pyramidal and octahedral Ni(II) ions, introducing extra paramagnetic metal centres which ‘switch on’ additional magnetic superexchange pathways. Subtle variations in the reaction scheme used to produce complexes 1 and 2 result in both a change of topology and an increase in nuclearity, through isolation of the hepta- and nonametallic complexes [Ni7(L1H)8(L1)2(H2O)6](SO4)·15H2O (3), [Ni9(μ-H2O)2(L2)6(L2H)4(H2O)2](SO4)·29H2O (4) and [Ni9(μ-H2O)2(L2)6(L2H)4(H2O)2](ClO4)2·2MeOH·18H2O (5) (where L2H2 = 2-(amino)phenylhydroxamic acid). Complementary dc magnetic susceptibility studies and DFT analysis indicate dominant antiferromagnetic exchange interactions in 1, 2, 4 and 5, but competing ferro- and antiferromagnetic exchange in 3.

 Please wait while we load your content...
Please wait while we load your content...