Azide trapping of metallocarbenes: generation of reactive C-acylimines and domino trapping with nucleophiles†
Abstract
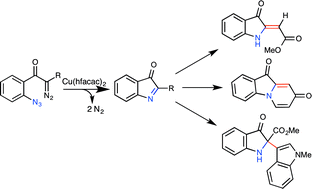
Azide-tethered diazocarbonyl compounds undergo copper-catalyzed conversion to transient C-acylimines. These reactive intermediates can be trapped with a variety of carbon nucleophiles, giving rise to complex 3-indolinone frameworks, including those with adjacent tetra-substituted carbon centers, in a single transformation.

 Please wait while we load your content...
Please wait while we load your content...