Calcite precipitation from by-product red gypsum in aqueous carbonation process†
Abstract
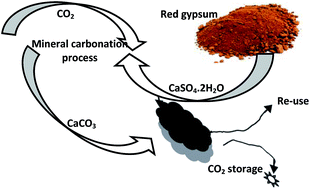
The carbon dioxide (CO2) concentration of the atmosphere has been increasing rapidly, and this rapid change has led to promotion of CO2 reduction methods. Of all the available methods, CO2 mineral carbonation provides a leakage-free option to produce environmentally benign and stable solid carbonates via a chemical conversion to a more thermodynamically stable state. In this research, the precipitation of calcite from by-product red gypsum was evaluated for mineral CO2 sequestration. For this purpose, the impact of changing variables such as reaction temperature, particle size, stirring rate, and liquid to solid ratio were studied. The results showed that optimization of these variables converts the maximum Ca (98.8%) during the carbonation process. Moreover, the results confirmed that red gypsum has a considerable potential to form calcium carbonate (CaCO3) during the CO2 mineral carbonation process. Furthermore, the low cost and small amount of energy required in the use of by-product red gypsum were considered to be important advantages of the CO2 sequestration process. Therefore, the acceptable cost and energy required in mineral carbonation processing of red gypsum confirms that using this raw material represents a method for mineral carbonation with minimal environmental impact.

 Please wait while we load your content...
Please wait while we load your content...