Chemoselective hydrogen peroxide oxidation of allylic and benzylic alcohols under mild reaction conditions catalyzed by simple iron-picolinate complexes†
Abstract
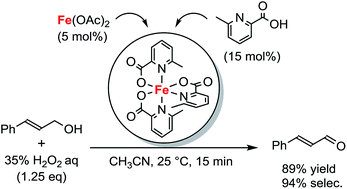
Chemoselective oxidation of allylic alcohols to α,β-unsaturated carbonyl compounds proceeded efficiently using hydrogen peroxide with iron-picolinate catalysts. The in situ generated [Fe(Me-Pic)3] (Me-Pic = 6-methylpicolinate) catalyzed oxidation of the alcohol moiety of primary allylic alcohols while the [Fe(Pic)3] (Pic = picolinate) and [Fe(Me-Pic)2(Pic)] did not show sufficient catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...