Cu(ii) and Co(ii) ternary complexes of quinolone antimicrobial drug enoxacin and levofloxacin: structure and biological evaluation†
Abstract
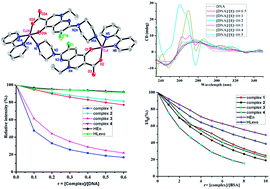
Four complexes of the quinolone antibacterial agent enoxacin (HEn) and levofloxacin (HLevo) with Cu2+ and Co2+ have been synthesized and characterized by physicochemical and spectroscopic techniques. Complex 1 is a novel dinuclear structure, in which enoxacin exhibits a tridentate binding mode bound to two Cu(II) ions through the pyridone oxygen, the carboxylate oxygen and the piperazine nitrogen atom, so far as we know such a mode and structure has never been presented before in metal–quinolone complexes. In mononuclear complexes 2–4, enoxacin and levofloxacin act as a bidentate ligand bound to the metal through the ketone oxygen and a carboxylate oxygen atom. The antimicrobial activity of the complexes 1–4 against four bacterial species were tested and the results indicated that they exhibit enhanced or similar activity to the free ligands. Studies on the interaction of 1–4 with calf-thymus (CT) DNA through UV and circular dichroism (CD) spectroscopies indicated that they bind to CT DNA probably by the intercalative binding mode. Fluorescence competitive studies with ethidium bromide (EB) revealed that the complexes could compete with EB and displace it to bind to DNA using the intercalative binding site. In addition, the complexes 1–4 exhibited good binding propensity to human and bovine serum albumin proteins with relatively high binding constants.

 Please wait while we load your content...
Please wait while we load your content...