Novel chiral tetramic acid-derived diols: organocatalytic facile synthesis and unique structural properties†
Abstract
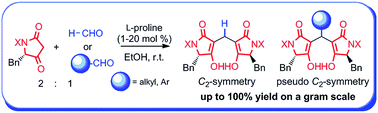
Organocatalytic tandem reactions of L-phenylalanine-derived tetramic acid with aldehydes allow a one-pot and high-yielding access to a diverse range of novel chiral diols in enantiomerically pure forms. In addition, a new entry of the diols, featuring their unique structures associated with C2- and pseudo C2-symmetric chiral motifs, is reported.

 Please wait while we load your content...
Please wait while we load your content...