Energy harvesting from humidity changes with a flexible coaxial electrode solid-state cell†
Abstract
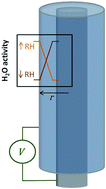
The response to humidity changes from the open-circuit potential (OCP) of a coaxial electrode polymer electrolyte cell was characterized. The OCP response showed a dependency on both the rate of change of the humidity and on the rate of water diffusion within the electrolyte, suggesting the Gibbs free energy due to ionic concentration gradients as the source of the OCP response. This is supported through the estimation of the membrane potential using the Nernst equation and the good agreement between the experimental and theoretical results. The method exploits the hygroscopic properties of the polymer electrolyte and utilizes changes in the relative humidity (RH) of the surrounding environment to induce a concentration gradient between the electrodes. The establishment of the concentration gradient is achieved through the use of a solid-state electrochemical cell with a coaxial electrode structure, enabling the exposure of only one of the electrodes to the environment. These results have implications for applications such as energy harvesting and self-powered sensors and additionally may provide a novel approach to studying the thermodynamic properties of polymer electrolytes.

 Please wait while we load your content...
Please wait while we load your content...