Effect of Al(iii) and curcumin on silk fibroin conformation and aggregation morphology
Abstract
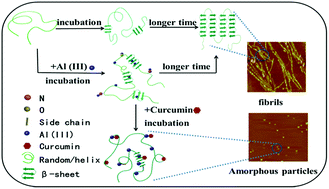
Misfolding or β-sheet nano-fibrillation of specific proteins is considered to be an underlying pathogenic mechanism of neurodegenerative diseases. It was found previously that Al(III) can affect the β-sheet nano-fibrillation and deposit neurodegenerative disease related proteins, and that curcumin can interact with metal ions and those proteins. In this work, silk fibroin (SF) was used as a model fibrillation protein for the investigation of the influence of Al(III) and curcumin on SF conformation transition. The effects of Al(III) and curcumin on SF were investigated using circular dichroism, thioflavin T fluorescence, 1-anilino-8-naphthalene sulfonate fluorescence, turbidity assays, atomic force microscope and Fourier transform infrared. This research demonstrated that Al(III) can bind with specific amino acid residues of SF, and then accelerate the formation of intermediates and also the formation of nanofibrils. The concentration of Al(III) is an important factor that influences the folding speed of SF. Furthermore, curcumin cannot only restrain the conformation transition of silk fibroin, but can also reverse the conformation transition of SF and Al(III)-induced SF from insoluble β-sheet to soluble random coil. Curcumin may prevent the neurodegenerative related proteins from nano-fibrillating and aggregating.

 Please wait while we load your content...
Please wait while we load your content...