S4N4 as an intermediate in Ag2S nanoparticle synthesis†
Abstract
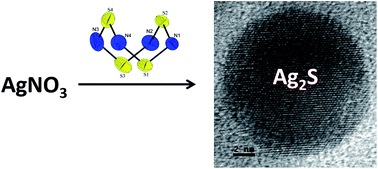
In the present work, hexamethyldisilazane assisted synthesis of Ag2S nanoparticles is demonstrated. Classical chemical investigations and nano investigations were utilized to explain the formation of nanoparticles. Controlled reactions were performed to explain the reaction mechanism. While establishing the reaction mechanism, the formation of Ag nanoparticles and tetrasulfur tetranitride (S4N4) was identified. Observations of a controlled reaction explained that sulfur reduction occurred through a S–N polymeric intermediate. Then, the polymeric intermediate was decomposed to tetrasulfur tetranitride. The formation of S4N4 was unambiguously confirmed by single crystal X-ray diffraction measurements. The formation of S4N4 also confirmed the role of hexamethyldisilazane as a reductant. Obtained nanoparticles were characterised by PXRD, EDAX, FTIR, FESEM, TEM, HRTEM and UV measurements.

 Please wait while we load your content...
Please wait while we load your content...