Efficient and scalable total synthesis of calcitroic acid and its 13C-labeled derivative†
Abstract
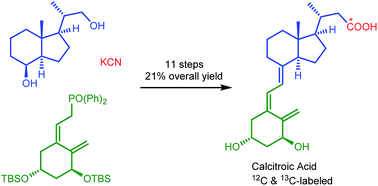
Calcitroic acid, the deactivated form of the physiological active vitamin D3 metabolite calcitrol, and its 13C labeled derivative has been synthesized starting from the commercially available Inhoffen-Lythgoe diol in 11 steps with an overall yield of 21%. The key steps in the synthesis were the formation of the C1-homologated nitrile with KCN and K13CN as well as the Horner–Wittig reaction with the ring A phosphine oxide reagent.

 Please wait while we load your content...
Please wait while we load your content...