Temperature-related reaction kinetics of the vanadium(iv)/(v) redox couple in acidic solutions
Abstract
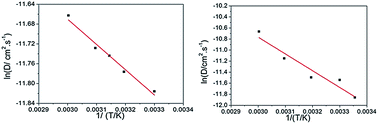
The temperature-related reaction kinetics of the VO2+/VO2+ redox couple on a graphite electrode in sulfuric acid solutions has been investigated by cyclic voltammetry and chronopotentiometry for a better understanding of positive half-cell in a practical vanadium redox flow battery (VRFB). Elevated temperature facilitates the VO2+/VO2+ redox reaction but without significant effect on its reversibility, and the reaction rate constants are calculated to be in the order of 10−3 cm s−1. In addition, the diffusion coefficient of both VO2+ and VO2+ ions tends to increase with increasing temperature with a diffusion activation energy of 4.24 kJ mol−1 for VO2+ and 25.2 kJ mol−1 for VO2+. On this basis, two diffusion equations for both VO2+ and VO2+ ions in sulfuric acid solutions have been established.

 Please wait while we load your content...
Please wait while we load your content...