One-pot route to β-adrenergic blockers via enantioselective organocatalysed epoxidation of terminal alkenes as a key step†
Abstract
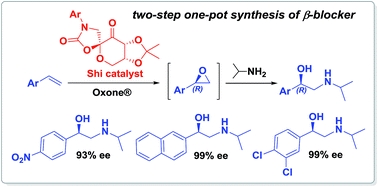
A convenient and environmentally attractive one-pot two-step process for the synthesis of β-adrenergic blockers via Shi's organocatalytic epoxidation of terminal alkenes and subsequent aminolysis reaction of epoxides with isopropylamine under mild reaction conditions has been developed.

 Please wait while we load your content...
Please wait while we load your content...