Inclusion complexes synthesized from an ABA triblock polymer and β-cyclodextrins: amplification of hydrophobic interaction along a hydrophilic polymer chain†
Abstract
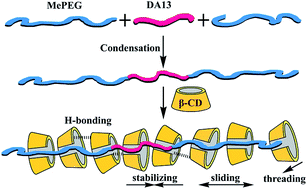
A triblock PEG-based polymer (CP1113) with a clear ABA structure was synthesized from 1,13-tridecanedioic acid (DA13) and mono-methoxy-poly(ethylene glycol) (MePEG) via condensation reaction. An inclusion complex (IC1113) with β-cyclodextrin (CD) was prepared by co-precipitation and the IC formation was confirmed by transmittance determination with a UV-Vis spectrometer. Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), and thermogravimetry analysis (TGA) were adopted to characterize IC1113 and the experimental data showed that a channel-type polypseudorotaxane was formed. For further investigating the inclusion behaviors, 1H-NMR spectroscopy was used to determine the molar ratio between CP1113 and β-CD and 2D ROSEY spectra were recorded to elucidate the IC formation mechanism. The results revealed that β-CD threaded onto the polymer main chain at a molar ratio of 7.8 : 1 and the molar ratio of EG segments to β-CD in IC1113 was ca. 8.28 : 1. It was concluded that β-CD accommodated both the methylene and the PEG segments and herein hydrophobic and hydrogen-bonds interactions played the most important roles with a hydrophobic stabilizing and hydrogen-bond inducing effect, which “amplified” the hydrophobic interaction between β-CD and the methylene segment along the hydrophilic PEG segments.

 Please wait while we load your content...
Please wait while we load your content...