Understanding the mechanism of the Povarov reaction. A DFT study†
Abstract
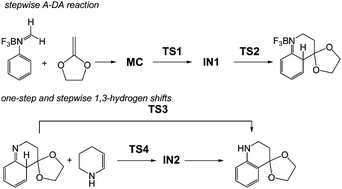
The molecular mechanism of the Povarov reaction in acetonitrile has been studied at the MPWB1K/6-311G** level of theory. This reaction follows a domino process that comprises two sequential reactions: (i) a Lewis acid catalysed aza-Diels–Alder (A-DA) reaction between a N-aryl imine and a nucleophilic ethylene yielding a formal [4 + 2] cycloadduct; (ii) a stepwise 1,3-hydrogen shift at this intermediate affording the final tetrahydroquinoline. At this computational level, the Lewis acid catalysed A-DA reaction presents a two-step mechanism as a consequence of the large stabilisation of the corresponding zwitterionic intermediate. Our study allows establishing that the N-aryl substituent has no remarkable incidence in the activation energy, but the presence of a second C-aryl substituent has a relevant role in the reaction rate. Analysis of the DFT-based reactivity indices of the reagents provides further explanation of the behaviours of the mechanism of the A-DA reaction involved in the Povarov reaction.

 Please wait while we load your content...
Please wait while we load your content...