The self-assembly and secondary structure of peptide amphiphiles determine the membrane permeation activity†
Abstract
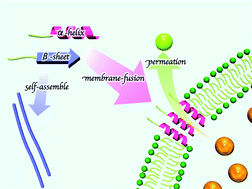
Membrane fusogenic peptides have attracted increasing attention because of their unique biofunctions in membrane translocation and viral infection. Here, we designed GALA-related peptides with palmitoyl tails. Our study indicated that the self-assembling propensity and the secondary structure of these peptide amphiphiles greatly influenced the membrane permeability.
- This article is part of the themed collection: In celebration of Seiji Shinkai's 70th Birthday

 Please wait while we load your content...
Please wait while we load your content...