Copper-catalyzed N-arylation of azoles and diazoles using highly functionalized trivalent organobismuth reagents†
Abstract
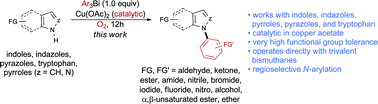
The N-arylation of indoles, indazoles, pyrroles, and pyrazoles using highly functionalized trivalent arylbismuth reagents is reported. The reaction is promoted by a substoichiometric amount of copper acetate, and it tolerates a wide diversity of functional groups on the azole and the organobismuth reagent. The method is also applied to the N-arylation of tryptophan derivatives.

 Please wait while we load your content...
Please wait while we load your content...