Synthesis and photochromic properties of benzofuran–phenanthrene and benzofuran–pyrene hybrids†
Abstract
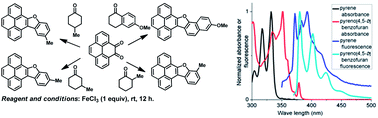
Iron(III) chloride mediated condensation of phenanthrene-9,10-dione and pyrene-4,5-dione with cyclohexanone and its derivatives furnish benzofuran–phenanthrene and benzofuran–pyrene hybrids. Absorption and emission spectroscopic studies revealed the bathochromic effect of benzofuran annulation on the photophysical properties of the pyrene chromophore. Solvatochromic studies revealed that solvent polarity did not have a noticeable effect on the absorption or the emission spectra. While benzofuran annulation enhanced the quantum yield in most of the solvents, appending a methyl group, in fact, reduced the quantum yield. Thus, our studies delineated the synthesis and photophysical properties of selected PAHs with a furan ring.

 Please wait while we load your content...
Please wait while we load your content...