On the non-additivity of the substituent effect in ortho-, meta- and para-homo-disubstituted benzenes†
Abstract
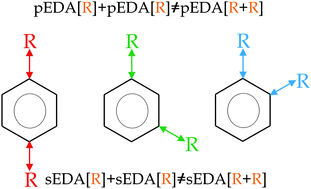
The non-additivity of the substituent effect in para-, meta- and ortho-homo-disubstituted benzenes was studied by means of the sEDA(I) and pEDA(I) substituent effect descriptors. The non-additivity effect on σ-valence orbitals is smaller than that on π-ones. For para- and ortho- substitution, the non-additivity effect on π-valence orbitals is ca. 2-times larger than that on σ-ones while for the meta-substitution, it is relatively small and similar in size. In general, there is: (i) the exponential-like increase of the non-additivity on the π-valence ring orbitals with the increase of pEDA(I), i.e., with substituent π-electron-donor properties; (ii) a lack of analogous correlations for the effect on the σ-orbitals; (iii) for para- and ortho-substituted benzenes, but not for meta-isomers, there is a rational-like concave downward decrease of the non-additivity of the σ-effect with an increase of the pEDA(I) descriptor. Thus, the non-additivity increases with the π-electron-donating character of the substituent, while the lack of a similar effect on σ-orbitals is connected to the locality of the σ-donating–accepting substituent effect. The decrease of the non-additivity of the sEDA(I) descriptor as the pEDA(I) descriptor is increased shows the presence of the hyperconjugation effect and the reorganization of the ring σ-electrons with the change of the π-electron systems.

 Please wait while we load your content...
Please wait while we load your content...