Electrochemical synthesis of ammonia from wet nitrogen using La0.6Sr0.4FeO3−δ–Ce0.8Gd0.18Ca0.02O2−δ composite cathode
Abstract
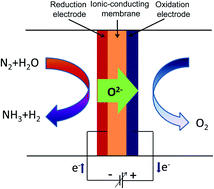
Electrochemical synthesis of ammonia from wet nitrogen in an electrolytic cell using a La0.6Sr0.4FeO3−δ–Ce0.8Gd0.18Ca0.02O2−δ composite cathode and an oxide–carbonate composite electrolyte has been investigated. La0.6Sr0.4FeO3−δ was prepared via a combined EDTA–citrate complexing sol–gel process, characterised by X-ray diffraction and SEM. A tri-layer electrolytic cell was fabricated by a one-step dry-pressing and co-firing process. Ammonia was successfully synthesised from wet nitrogen under atmospheric pressure. Ammonia formation was observed at 375, 400, 425 and 450 °C and the maximum ammonia formation rate of 7 × 10−11 mol s−1 cm−2 was observed at 400 °C when a voltage of 1.4 V was applied. This ammonia formation rate corresponds to a Faradaic efficiency of ∼0.14% at a current density of 14.25 mA cm−2.

 Please wait while we load your content...
Please wait while we load your content...