A novel Gaussian-DAEM-reaction model for the pyrolysis of cellulose, hemicellulose and lignin
Abstract
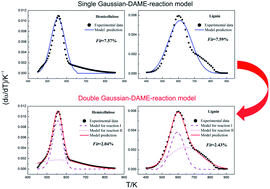
This work deals with the pyrolysis kinetics of cellulose, hemicellulose and lignin. Experiments were carried out in a thermogravimetric analyzer (TGA Q5000) in the inert atmosphere of nitrogen at a heating rate of 10 K min−1. The single Gaussian distributed activation energy model (DAEM) was utilized to study the pyrolysis kinetics. Kinetic parameters such as the pre-exponential factor (k0), mean activation energy (E0) and standard variance (σ) were computed by a pattern search algorithm. It was found that the calculated kinetic parameters using the single Gaussian-DAEM-reaction model could reproduce the differential thermogravimetric (DTG) curves of cellulose very well. However, there existed obvious deviations in the whole range of temperatures between the calculated and experimental data for hemicellulose and lignin. In order to describe the thermal decompositions of hemicellulose and lignin more accurately, a novel double Gaussian-DAEM-reaction model consisting of two parallel partial reactions was developed to describe the pyrolysis processes. Calculated results of the fitting procedure using the double Gaussian-DAEM-reaction model showed good agreement with experimental DTG data of hemicellulose and lignin.
- This article is part of the themed collection: Computational chemistry

 Please wait while we load your content...
Please wait while we load your content...