Bis-triazolium containing macrocycles, pseudorotaxanes and interlocked structures for anion recognition†
Abstract
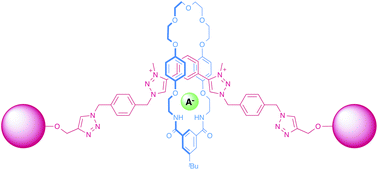
Two bis-triazole macrocycles were prepared by the copper(I)-catalysed azide alkyne cycloaddition of a bis-azide and 1,6-heptadiyne or 1,3-diethynylbenzene. Upon alkylation of one of these to give the dicationic bis-triazolium cycle, a potent receptor was generated which was demonstrated to be selective for sulfate. Two acyclic bis-triazolium receptors were also synthesized and the halide anion recognition properties of these systems investigated, as well as their ability to form anion templated pseudorotaxanes with an isophthalamide macrocycle. Interestingly, the propyl-linked bis-triazolium host displayed stronger anion binding affinities than the analogous 1,3-phenyl-linked system, although the phenyl-linked receptor formed a more stable halide anion templated pseudorotaxane. An acyclic bis-triazolium threading component produced a stable sulfate anion templated pseudorotaxane with a bis-triazolium macrocycle, which was used to synthesise a tetra-triazolium catenane. An anion templated stoppering strategy was used to prepare a bis-triazolium rotaxane host system which binds halide anions selectively over dihydrogenphosphate in competitive 1 : 1 CDCl3 : CD3OD solvent media.
- This article is part of the themed collection: Supramolecular chemistry: self-assembly and molecular recognition

 Please wait while we load your content...
Please wait while we load your content...