Rh2(OAc)4-catalyzed 2,3-migration of β-ferrocenecarboxyl α-diazocarbonyl compounds: an efficient synthesis of ferrocene-containing α,β-unsaturated esters†
Abstract
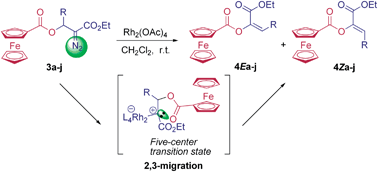
A series of β-ferrocenecarboxyl α-diazocarbonyl compounds were prepared by the reaction of ferrocenoyl chloride with β-hydroxyl α-diazocarbonyl compounds in the presence of pyridine. The diazo decomposition of these ferrocene-containing diazocarbonyl compounds with Rh2(OAc)4 resulted in 2,3-ferrocenecarboxyl migration to give ferrocene-containing α,β-unsaturated esters in high yields.

 Please wait while we load your content...
Please wait while we load your content...