Enhancing catalytic activity and stability for CO2 methanation on Ni–Ru/γ-Al2O3via modulating impregnation sequence and controlling surface active species
Abstract
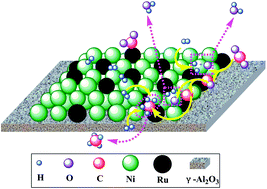
Bimetallic Ni–Ru NPs loaded onto γ-Al2O3 were prepared by co-impregnation and sequential impregnation methods and were investigated for CO2 methanation in the temperature range of 250–500 °C under atmospheric pressure to uncover the dependence of activities on surface species. It was found that the activities of CO2 methanation were very highly dependent on the preparation sequence. Comparing the activity results dependence with the characterization results of XRD, H2-TPR, BET, TEM-EDX and XPS techniques, it was indicated that the dispersion of Ru and Ni was controlled by preparation steps, and the tendency of Ru segregation on the catalyst surface was identified. This tendency led to the high activity of the 10Ni–1.0Ru catalyst. Such surface segregation phenomena of Ru in the co-impregnation process controlled the chemical state of surface Ru species, allowing them to be easily reduced to the metallic state. In addition, the 10Ni–1.0Ru catalyst performed with high stability and showed almost no deactivation up to 100 h in long term stability tests. The possible reaction mechanism was proposed, in which CO2 was dissociated and was activated on Ru species' surfaces to form carbon species (COads), and then was reacted with activated H on Ni centres to form methane.

 Please wait while we load your content...
Please wait while we load your content...