Switchable fluorescence by click reaction of a novel azidocarbazole dye†
Abstract
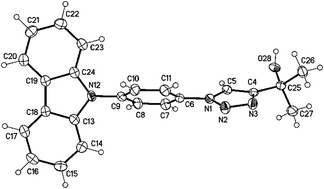
Imaging is – even these days – still restricted to of a few classes of robust dyes. A demand for switchable tags led us to the design of a new class of pre-fluorophores. We achieved this by using a non-fluorescent N-(4-azidophenyl)-carbazole tag which turns fluorescent by click reaction with alkynes and cyclooctynes. The spectral properties of the labelled dyes were investigated. Our results suggest that a twisted internal charge transfer (TICT) transition is responsible for the emission. DFT calculations and single-crystal X-ray diffraction of selected examples support this explanation. The feasibility of the new dyes for biological application has also been tested via confocal microscopy.

 Please wait while we load your content...
Please wait while we load your content...