Hydrogenation of CO on interstellar dust: what is the role of water molecules?
Abstract
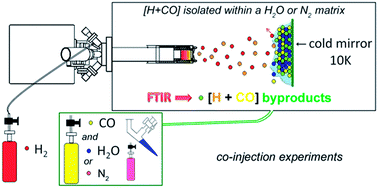
We performed several co-injection experiments varying the concentration of water molecules within a binary mixture (CO/H2O) co-condensed with (H/H2) onto a cold mirror maintained at 10 K. We performed the [CO + H] reaction within a H2O-rich and a N2-rich environment and confirmed that the gradual replacement of CO molecules by water molecules greatly enhances the yields of H2CO and CH3OH, the main products of CO hydrogenation at 10 K. This trend is observed until the concentration of CO molecules becomes too low and significantly favors the side reaction [H + H] recombination over CO hydrogenation. Irradiation of (CO/H2O) mixed ices with cold H-atoms highlights the action of water molecules, expressed in the spectra as a decrease in the intensity of water-ice's infrared signatures. The catalytic effect observed can be interpreted by physical, chemical, and energetic considerations. This work is inclined toward extending previously reported results with different experimental conditions.

 Please wait while we load your content...
Please wait while we load your content...