Lignin depolymerization via an integrated approach of anode oxidation and electro-generated H2O2 oxidation†
Abstract
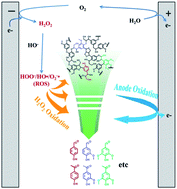
Lignin is a natural aromatic macromolecule in huge quantity and might serve as sustainable resources for the chemical industry after being depolymerized. An electrochemical approach combining anode oxidation and electro-generated H2O2 oxidation has been developed for converting lignin into value-added aromatic chemicals in this study. Lignin in alkali solution was electrolyzed in an undivided electrolytic cell with a cylindrical graphite felt cathode and a RuO2–IrO2/Ti mesh anode, in which the by-product O2 on the anode could be efficiently reduced to H2O2 on the cathode in situ. Results display that the depolymerization productivity via the integrated approach obviously surpassed the sum of that by separate H2O2 oxidation and anode oxidation. Moreover, the analysis results of GC-MS, GPC, and C9 expanded formula confirmed that C–C bonds and C–O–C bonds in lignin were cleaved synergistically by direct anodic oxidation and indirect H2O2 oxidation, and the macromolecules are gradually depolymerized into final products of monomers and dimers.

 Please wait while we load your content...
Please wait while we load your content...