Tetrabutylammonium bromide-mediated ring opening reactions of N-tosylaziridines with carboxylic acids in DMF†
Abstract
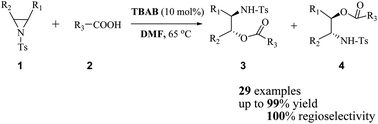
Tetrabutylammonium bromide (TBAB) was found to be a highly effective catalyst at as low as 10 mol% for regioselective ring opening of a variety of N-tosylaziridines with various carboxylic acids to afford the corresponding ring-opening products (derived β-amino esters) in good to excellent yields and up to 100% regioselectivity in combination with DMF as the solvent.

 Please wait while we load your content...
Please wait while we load your content...