Peptide nucleic acid–nanodiamonds: covalent and stable conjugates for DNA targeting†
Abstract
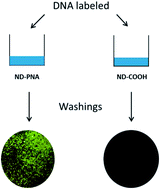
This article highlights our recent application of functional nanodiamonds (NDs) with peptide nucleic acids (PNA) to develop tools for DNA detection. NDs appear as an ideal nanocarrier due to their versatile surface chemistry, their non-cytotoxicity and since they could benefit from intrinsic luminescent properties. In this work, we report for the first time the possibility to prepare a covalent, stable and functional conjugate of PNA with 20 nm HPHT (High Pressure High Temperature) nanodiamonds. Peptide nucleic acid is a DNA mimic related to both peptides via its backbone and to nucleic acid via its bases. It binds more specifically and more strongly than DNA itself to either DNA or RNA. We have initiated a novel functionalization route based on an optimized amidation of ND carboxylic acid groups, to produce ND–PNA conjugates via an efficient, simple and reproducible method. We describe the synthesis and characterization of those conjugates. The covalent binding of the ND–PNA and the loading of nucleic acid grafted onto the NDs were performed using various characterization methods including FTIR, Kaiser tests and thermogravimetry. Then, ND–PNA conjugates were validated through a successful recognition of complementary DNA in a mixture, showing their efficiency toward nucleic acid detection. Moreover, the impact of ND–PNA on A 549 cells’ viability was analysed with flow cytometry and showed an absence of ND–PNA conjugates cytotoxicity. Such nucleic acid-functionalized nanodiamonds offer a wide range of applications and namely the possibility to target and to recognize DNA.

 Please wait while we load your content...
Please wait while we load your content...