The effect of oxygen vacancies and fluorine dopant over adsorption behaviours of V2O5/TiO2 for NO removal
Abstract
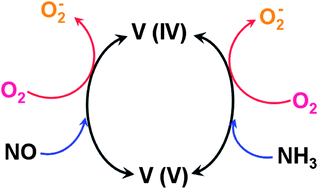
Oxygen vacancies are omnipresent on an oxide surface under ambient conditions. The addition of fluorine to V2O5/TiO2 increases the number of oxygen vacancies which can react with O2 to form a superoxide species. The adsorption of NO and NH3 on the surface of the F-doped V2O5/TiO2 catalyst is investigated by electron paramagnetic resonance (EPR), X-ray photoelectron spectroscopy (XPS) and temperature programmed desorption (TPD). Active oxygen species formed on the surface of the catalyst are detected by EPR. At 513 K, the adsorption of NO and NH3 possibly lies in the vicinity of the surface superoxide radicals, and leads to a change in the nearby electronic structure of these sites. The present results show that F-doping can form more oxygen vacancies on the surface of the catalyst. The oxygen vacancies play an important role in the catalytic conversion of the nitrogen oxides, because they can improve the adsorption and activation of NO, NH3 and O2. Additionally, the results of NO-TPD and NH3-TPD demonstrate that there is a close correlation between the adsorption amounts of NO or NH3 and the oxygen vacancy concentrations of the catalysts. The stability and lifetime of the surface O2− anions are directly correlated to the structure of the adsorption site on the catalyst surface and influence the catalytic ability of the catalyst to adsorb reaction gases under the NH3-SCR operating conditions.

 Please wait while we load your content...
Please wait while we load your content...