The influence of protecting polyelectrolyte layers on the temperature behavior of NaBD4
Abstract
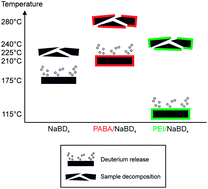
The use of NaBH4 as a hydrogen storage material suffers to some extent from its deficient stability against chemicals and degradation at elevated temperatures. This disadvantage can be overcome by the use of polyelectrolytes as protective layers. Furthermore, the coating of NaBH4 with polyelectrolytes significantly enhances the release of hydrogen from the storage material. In this work, the influences of polyethyleneimine (PEI) and poly(acrylonitrile-co-butadiene-co-acrylic acid), dicarboxy terminated (PABA) as protective polyelectrolytes coatings have been investigated on deuterated sodium borohydride, thus being able to determine hydrogen release from the polyelectrolyte and the hydrogen storage material. The release rates have been investigated by temperature-programmed desorption measurements of significant species as preliminarily identified by mass spectrometry. Furthermore, the geometrical structures of the polyelectrolyte films were characterized by confocal laser scanning microscopy studies prior and posterior to the temperature treatment.

 Please wait while we load your content...
Please wait while we load your content...