Microwave selective heating-enhanced reaction rates for mullite preparation from kaolinite
Abstract
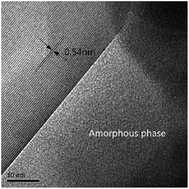
Mullite is becoming increasingly important for use in refractory lining, structural ceramic and catalyst products. The kaolinite-mullite process is a simple method to prepare mullite. Under conventional heating kaolinite has to be heated at 1400 °C for 1 h to form well-ordered orthorhombic mullite (3Al2O3·2SiO2) that is accompanied by the formation of cristobalite. However, under microwave heating at 900 °C for 5 min and 1200 °C for 1 min, respectively, kaolinite transformed to well-ordered orthorhombic mullite without the formation of cristobalite. The ultra-fast heating rate of microwaves, due to selective heating, accelerated the nucleation and growth of mullite grains and changed the mullitization route of kaolinite. Before mullitization, the capacity for microwave absorption of decomposed kaolinite is dependent on the amount of structural OH up to a temperature of 522 °C and is then dependent on the amount of metakaolinite, gamma alumina and amorphous silica that are present in the sample. The dielectric loss of pseudo-tetragonal mullite has the highest value of the dielectric losses for all of the phases that result from the kaolinite-mullite process.

 Please wait while we load your content...
Please wait while we load your content...