Asymmetric hydrogenation of 3-substituted 2H-1,4-benzoxazines with chiral cationic Ru-MsDPEN catalysts: a remarkable counteranion effect†
Abstract
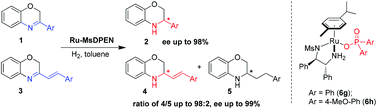
The enantioselective hydrogenation of 3-aryl and 3-styryl-substituted-2H-1,4-benzoxazines was developed using the chiral cationic Ru(η6-cymene)(MsDPEN)(Ar2PO2) system in high yields with up to 99% ee. The counteranion was found to be critically important for the high enantioselectivity. Furthermore, the regioselectivity could be regulated by the counteranion of the catalyst in the asymmetric hydrogenation of 3-styryl-2H-1,4-benzoxazines.

 Please wait while we load your content...
Please wait while we load your content...