Palladium-catalyzed, copper-mediated construction of benzene rings from the reactions of indoles with in situ generated enones†
Abstract
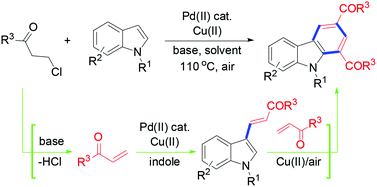
Construction of the benzene ring in carbazoles was efficiently realized through a domino dehydrochlorination/alkenylation/cycloaddition–oxidation sequence by means of palladium(II)-catalyzed, copper(II)-mediated reactions of N-protected 2,3-unsubstituted indoles with 3-chloropropiophenones in the presence of a base. 3-Alkenylated indole was confirmed to be formed as the reaction intermediate which then underwent Diels–Alder cycloaddition to the initially in situ generated enone from a 3-chloropropiophenones substrate, and the subsequent dehydrogenative aromatization yielded the carbazole product. The strategy to employ in situ generated enones as the reactive species avoided the use of a large excess of labile substrates and lessened the side reactions.

 Please wait while we load your content...
Please wait while we load your content...