Palladium-catalyzed reductive cleavage of tosylated arenes using isopropanol as the mild reducing agent†
Abstract
The deoxygenation of tosylated arenes catalyzed by a palladium complex is described. This method represents one of the first general examples of reductive C–O bond cleavage of aryl tosylates via palladium catalysis. By simply employing isopropanol as a mild reducing agent, a variety of tosylated arenes can be smoothly reduced. Labelling experiments revealed that the H source is isopropanol.
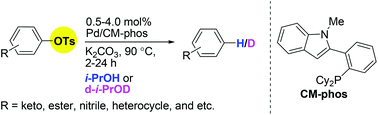
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers for 2014

 Please wait while we load your content...
Please wait while we load your content...