A series of novel mercury(i) selenites and tellurites containing SOJT Mo6+ cations†
Abstract
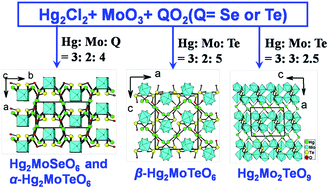
Using Hg2Cl2, MoO3, and TeO2 (or SeO2) as starting materials, four new mixed-metal tellurites and selenites have been obtained in the unexplored mercury(I)–Mo6+–Se4+/Te4+–O system, namely, Hg2MoSeO6 (1), α-Hg2MoTeO6 (2), β-Hg2MoTeO6 (3), and Hg2Mo2TeO9 (4). They represent the first mercury(I) tellurites and selenites containing octahedrally coordinated d0 transition metal (TM) cations. All four compounds are centrosymmetric. They show three types of 3D structures based on Hg22+ dumbbells, MoO6 octahedra, QO32− (Q = Se, Te) or TeO44− anions. Compounds 1–3 feature similar 1D anionic chains of [MoO3(QO3)]2− (Q = Se or Te), in which the 1D chains of corner-sharing MoO6 octahedra are further decorated by bidentate bridging selenite or tellurite anions. Compounds 1 and 2 are isostructural and their 3D network structures are based on 2D mercury tellurite or selenite layers in the ac plane pillared by MoO6 octahedra, whereas the 3D network of β-Hg2MoTeO6 (3) is composed of 1D mercury tellurite chains along the a axis and 1D chains of [MoO3(TeO3)]2− anions along the b axis. Compound 4 features an unusual 3D network structure composed of novel [Mo2O5(TeO4)]2− double layers interconnected by Hg22+ cations. Within the [Mo2O5(TeO4)]2− double layer, the cyclo-Mo4O20 tetramers are further interconnected via corner sharing into a 2D molybdenum oxide double layer with TeO4 groups capping the walls of the thus-formed eight-membered rings (8-MRs). Thermal stabilities and optical properties as well as theoretical calculations based on density functional theory (DFT) methods were also performed.
- This article is part of the themed collection: Crystal engineering for molecular materials

 Please wait while we load your content...
Please wait while we load your content...