Activated lipidic cyclic carbonates for non-isocyanate polyurethane synthesis†
Abstract
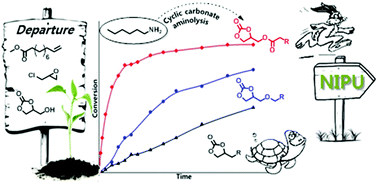
Activated 5-membered cyclic carbonates were prepared from glycerol and fatty acid derivatives. Ester and ether moieties were introduced in the β position to the cyclic carbonate, in order to enhance its reactivity towards amines. 1H NMR kinetic investigation of the aminolysis of these cyclic carbonates demonstrated a higher reactivity compared to the one of alkyl substituted cyclic carbonates. In the case of ester-activated carbonates, a reactivity similar to the one of 6-membered ring cyclic carbonates was observed. Moreover, these carbonates exhibited amidation side-reactions with amines that could be however prevented by decreasing the temperature to room temperature. Poly(hydroxyurethane)s (PHUs) were then synthesized from these activated 5-membered ring cyclic carbonates at 70 °C in DMF (1 mol L−1) and exhibited molar masses up to 13 700 g mol−1 with Tg in the range −26 to −10 °C.

 Please wait while we load your content...
Please wait while we load your content...